

On day someone told a friend that on their hillside property a thin layer of quartz outcropped, forming a white, shiny, knee-high bench across the field. The friend wanted to see this, so off they went. When the outcrop was located, the friend got onto his knees and with a hand lens looked closely at the white rock. Seeing what's shown in the picture at the right, he stood up and announced, "It's calcite, not quartz."
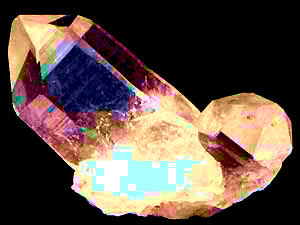
It was calcite and not quartz because of features of the partially exposed crystal in the above picture. Quartz crystals are six-sided, with their sides connecting with one another at 120° angles, as the quartz crystal at the left shows. The above crystal is rhombohedral. Rhombohedrons have opposite sides parallel with one another, and the angles where the sides meet are either more or less than 90°, as displayed by the above calcite crystal. Moreover, it made sense for calcite to be outcropping on the hillside because all the bedrock in that region was limestone, and limestone is mostly calcium carbonate, CaCO3, which is the mineral calcite.
What are minerals?
Minerals have fairly definite chemical formulas and in their natural state usually occur in much smaller quantities than "rock size." Rocks are composed of one or more minerals in varying proportions.
In geology, minerals are solid chemical compounds with a fairly well-defined chemical composition and, when in pure form, forming crystals of a specific structure.
Below, a sedimentary limestone rock is seen coated with the rusty-red mineral known as cinnabar. It was found next to an abandoned mercury mine, which makes sense because cinnabar is a mineral with the chemical formula HgS, composed of the elements mercury (Hg) and sulfur (S).

On the rock, notice that no crystals are apparent. That's normal because in Nature minerals tend to mix together, creating mixtures in which not enough of the right kinds of molecules can gather in one place for easy-to-see crystals to form. Sometimes crystals that do form are too small to see. Other times, maybe large crystals form, but they fuse together forming massive rock.

For example, at the right is a close-up of a granite rock. Notice the rock's lumpiness. The larger white masses are crystaline quartz, SiO2, and orthoclase feldspar, KAlSi3O8. Crystals aren't seen because minerals other than quartz disrupted the crystals' growth, and crystals that did form fused with the matrix. The dark spots are various dark minerals.
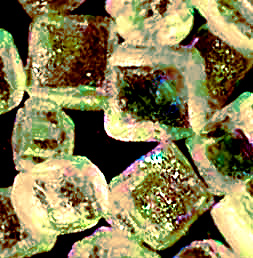
Rock salt, technically known as halite, NaCl, is formed in a similar matter, with "impurities" disrupting crystal growth, and much fusion. To accomplish the tiny, cubical crystals of table salt shown at the left, salty water is evaporated, and the resulting slurry is dried, sieved and graded, ending up with table salt.
8 GREAT CLASSES OF MINERALS
The more than 5,000 kinds of minerals are categorized in various ways. Probably the Dana System is the most commonly used, and here it is, with example mineral names in
- Native Elements {single elements naturally occurring in pure form}
- metals (
gold Au ,copper Cu , etc.) - semi-metals (
arsenic As ,tellurium Te , etc.) - non-metals (
sulfur S ,diamond C , etc.)
- metals (
- Sulfides {compounds of sulfur (S) with one or more metals}
galena PbS ,pyrite FeS2 , etc.
- Oxides & Hydroxides {metal combined with oxide anion (O2−), water (H2O) or hydroxyl (OH)}
hematite Fe2O3 ,corundum Al2O3 , etc.
- Halides {mineral with fluorine (F), chlorine (Cl), bromine (Br), or iodine (I)}
halite (salt) NaCl ,fluorite CaF2 , etc.
- Carbonates, Nitrates & Borates {with the carbonate ion (CO32-), nitrate ion (NO3-), or borate (BO3) unit}
calcite CaCO3 ,dolomite CaMg(CO3)2 , etc.
- Sulfates, Chromates, Molybdates {with sulfate ion (SO42-), the chromate radical (CrO4), or the molybdate radical (MoO4)}
gypsum CaSO4•H2O ,anhydrite CaSO4 , etc.
- Phosphates, arsenates, vanadates {with phosphate (PO43-), arsenate (AsO43-), or vanadates (VO43-) or higher anion group}
turquoise CuAl6(PO4)4(OH)8•H2O , etc.
- Silicates (any of many silicon-oxygen compounds)
quartz SiO2 ,opal SiO2•nH2O , etc.
The usefulness of being familiar with the eight great classes of minerals is that within each family the many minerals may share certain features. If an unfamiliar mineral seems to belong to a certain class, by remembering general features of minerals in that class, you can make educated guesses as to its probability of occurring in particular geological settings, its physical properties, etc.
MICA: A SAMPLE MINERAL

Let's say you're in southern Canada where the Canadian Shield outcrops and you find the flat, shiny rock at the right. It's mica, one of over 5000 minerals, and can serve as an example of what it can be like getting to know any mineral.
First we learn that the word "mica" applies to a group of minerals, not just one. The outstanding physical characteristic all the micas is that individual mica crystals can easily be split into extremely thin elastic plates. Often thin sheets of mica are easy to separate from one another -- they easily exfoliate.
 Mica window in Russia, 1600s; State Historical Museum; image courtesy of 'Shakko' via Wikimedia Commons
Mica window in Russia, 1600s; State Historical Museum; image courtesy of 'Shakko' via Wikimedia CommonsIn fact, before glass became easy to manufacture, big sheets of mica often served as windowpanes, as seen at the left. Today mica still is used in other ways because both heat and electricity have a hard time passing through it. Because mica maintains its electrical insulation properties even at very high temperatures, it's used in delicate machinery where there's high voltage, such as turbo-generators and electrical motors.
Mica belongs to the silicate class of minerals, so it's composed mostly of SiO2. The formula for muscovite, or common mica, is KAl2(AlSi3O10)(F,OH)2, or (KF)2(Al2O3)3(SiO2)6(H2O), depending on the local geological environment. It's a bit unusual to have such a variable, complex formula, but with 5000 minerals, occasional surprises are to be expected. In nature, the micas form veins in rock, typically in association with other silicate minerals, particularly quartz and feldspar.
The two best known mica varieties are:
- Muscovite can range from transparent to clear yellow, brown, green or red; potassium and aluminum; the main non-silicate elements in it are potassium and aluminum
- Biotite ranges from black to dark green or brown, though sometimes it can be pale yellow; the main non-silicate elements in it are potassium, magnesium, iron and aluminum
India, China, the US, South Korea and Canada possess the greatest deposits of mica.
WHAT CAN WE DO WITH MINERALS?
Most of us live within easy travel distance of where rocks, gravel or sand are exposed, and most of us travel sometimes, so there's plenty we can do with minerals, starting with identifying what we find.
For identification, we need to acquire a field guide to mineral identification. You can see what's available by doing an Internet search on the keywords "book mineral identification."
MICA:
color: several colors
streak: when scratched across a hard, white plate, white
luster: pearly
diaphaneity: transparent to translucent
cleavage: flat sheets
hardness: 2 to 4
specific gravity: 2.7 to 3
crystal system: monoclinic)
You can look up a mineral's properties on Wikipedia's List of Minerals page.
At the left we see a list of the main physical features of our sample mineral mica. All these characteristics and sometimes more are useful in determining any mineral's identity. At the right you see a commercial "mineral identification kit." It's interesting to see what the kit makers think is necessary for mineral identification.
The kit most importantly includes, besides 20 sample minerals, the following:
- a magnifying glass, especially for seeing crystal structure.
- a streak plate on which to scratch your mineral to see the color left on the plate
- magnet (some minerals are attracted)
- iron nail, presumably for determining the mineral's hardness
 With hand lens and geology hammer, NASA astronauts practice for geology on the Moon; image courtesy NASA
With hand lens and geology hammer, NASA astronauts practice for geology on the Moon; image courtesy NASAAlso needed would be a small bottle of weak acid to see if the mineral fizzes when the acid is applied. Quartz doesn't fizz, but calcite does. A notebook is needed, and a geology hammer is always convenient for breaking off samples.
So, by starting a mineral collection and identifying your finds, you need to notice lots of features and have certain understandings, the accomplishment of which will keep you busy for a long time.
You might enjoy seeing part of the Smithsonian Gem & Mineral Collection. Also, you can see many remarkable minerals for sale at the ETSY commercial site where many remarkable minerals are for sale.