
Here's a story showing how knowing about elements can be useful for backyard naturalists/rockhounds:
Maybe you find the rock pictured at the right. In your field guide you match your find with a picture of "banded chert," which the book says is composed of microscopically crystalline quartz. Quartz, you read elsewhere, "is the second most abundant mineral in Earth's continental crust, behind feldspar." Therefore our fancy rock isn't particularly rare or unusual, but, still, the banding is a pretty way for its impurities to arrange themselves, so we take it home to add to our collection.

But, back at the house, we see that we've already found something very similar to our banded chert, shown at the left, but we'd identified that as "banded agate." This jogs our memory. Back when that banded agate was found, we'd learned that rocks called chert, flint, agate and chalcedony all are composed of microscopically crystalline quartz, and they intergrade with one another. Agate and chalcedony tend to be glassy and translucent, instead of dull like our chert rock. Flint tends to be darker than chert. Rockhounders sometimes debate where the boundaries are, but to you the banded chert isn't glassy and translucent enough to be agate or chalcedony, and it's too pale to be flint. Your agate, though, is only somewhat glassy, so...
Whatever the case with your agate, how can it be that chert, flint, agate and chalcedony all are composed of microscopically crystalline quartz with a few impurities, yet they're different enough to have their own names and characteristics? Plus, most of us know that quartz crystals such as the one shown the right are hard, glassy and transparent, very unlike any form of chert, flint, agate and chalcedony. How come?
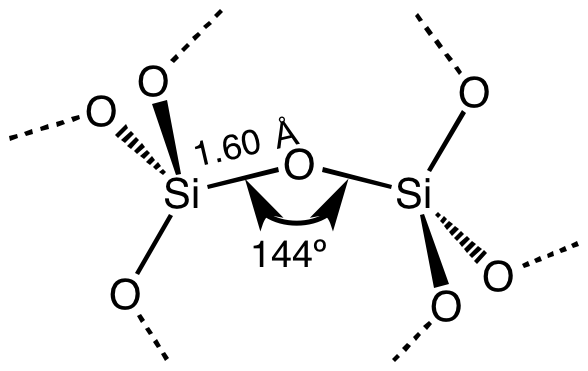 fragment of most SiO2 polymorphs; diagram courtesy of 'Smokefoot' via Wikimedia Commons"
fragment of most SiO2 polymorphs; diagram courtesy of 'Smokefoot' via Wikimedia Commons"To begin with, quartz is a mineral with the chemical composition of SiO2. In a molecule of SiO2, one atom of silicon (Si) bonds with two atoms of oxygen (O) as shown at the left (solid narrow lines). Through the convention of using different kinds of lines showing different kinds of bonding between the silicon and oxygen atoms, the diagram indicates that the silicon atoms not only interact with their two closest oxygen atoms, but also with nearby oxygen atoms. Moreover, the diagram shows one morph, or form, of SiO2, which is "polymorphic," meaning that SiO2 (quartz) can exist with more than one internal molecular structure. The distance between atoms -- represented in Angstroms (Å, 10-10m) -- but also the relative angle by which oxygen atoms bond with their silicon atom can vary.
AND ASTRONOMY
 If you need to review the basic facts about electrons, protons, atoms, molecules, elements, and the like, a good place to begin is ThoughtCo.Coms General Chemistry Page. NASA has an interesting page on "Where Your Elements Came From."
If you need to review the basic facts about electrons, protons, atoms, molecules, elements, and the like, a good place to begin is ThoughtCo.Coms General Chemistry Page. NASA has an interesting page on "Where Your Elements Came From."
In other words, SiO2's three-dimensional matrix can be squeezed and warped enough (changing distances between atoms, and the angles of their bonds) to make room for atoms of impurities (such as calcite, iron oxides, clay minerals, etc.), providing variations in color, opacity, hardness and other features. The above quartz crystal is hard, glassy and transparent because its atoms are as orderly packed together as possible, without disruptive impurities. A chert rock's "microcrystalline" particles may be a mixture of microscopic but pure, transparent quartz crystals reflecting light in all directions, causing the rock to be opaque and white instead of transparent or translucent.
MINERALS FROM STARS
Big Bang
Large Stars
Super-novae
Cosmic Rays
Small stars
Man-made
NASA's "Astronomy Picture of the Day" for January 25th, 2016, though just a chart instead of the usual gorgeous pictures of outer space normally featured, is as thought-provoking as any picture of a spiraling galaxy or all-consuming black hole. The chart, adapted from the normal chemistry-class Periodic Table of Elements listing the 118 known chemical elements, shows where each element originally came from. All known possible sources are listed at the left
46% oxygen
28% silicon
8.3% aluminum
5.6% iron
4.2% calcium
2.5% sodium
2.4% magnesium
2.0% potassiume
0.61% titanium
<0.15% all other elements
All naturally occurring elements originated during the Big Bang, in stars or exploding stars, or maybe something else out in the Universe (The source of cosmic rays isn't quite clear).

So, the rocks and minerals we pick up all consist of minerals consisting of elements from outer space. The elements and minerals which coagulated here on Earth interacted with one another, and were pressurized and depressurized, heated and cooled, and given a place to rest awhile. That pumice rock floating on water at the left, like our chert and agate, is mostly the mineral quartz, SiO2, with aluminum oxide and trace amounts of other oxides. Pumice is so unlike our other mostly-SiO2 rocks because of its history of its elements and minerals interacting with one another, pressurizing and depressurizing, heating and cooling, and it's grand being able to visualize all this when one day the rock comes floating along shore!